Enzymes: Grade 9 Understanding for IGCSE Biology 2.10 2.11 2.13
Enzymes are biological catalysts. This means they are able to increase the rate of a chemical reaction but are not used up in the reaction. Without enzymes the reactions of metabolism would all happen too slowly for life to exist – enzymes can speed up the rate of reaction by many millions of times….
Enzymes work as catalysts by lowering the activation energy needed for the reaction to occur. Activation energy is the term for the extra energy needed to be given to the reactants to break bonds within them to allow the product molecules to be formed. Enzymes provide an alternate reaction pathway that has a lower activation energy. This means that under any conditions a higher proportion of the reactant molecules will have sufficient energy to overcome the activation energy barrier and so more reactants will be turned into products.
How do enzymes lower the activation energy?
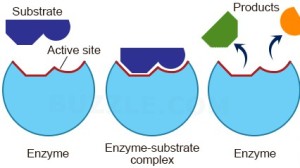
Enzymes are all large globular molecules, almost always made of protein. They have a specific three-dimensional shape that includes a region called the active site which has a shape that allows the reactant molecules (called substrates) to bind. When the enzyme binds to the substrate, it forms an enzyme-substrate complex.
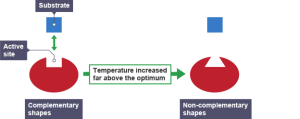
This theory of how enzymes might work is called the Lock and Key theory. The active site acts like a lock as it has a shape that is complementary to the shape of the substrate (the key). Lock and Key theory explains an important property of enzymes which is that they are specific. Each enzyme can only catalyse one reaction since only a substrate molecule with a specific shape can bind to the active site.
When the substrate is bound to the active site forming an enzyme-substrate complex, the enzyme introduces a strain on some of the bonds in the substrate, making a reaction more likely. The active site might provide a microenvironment that is exactly the right condition for the reaction, thus lowering the activation energy.
Key idea: enzymes catalyse almost all the chemical reactions that happen in organisms. It is easy to imagine that enzymes only catalyse reactions like the one in the picture above in which a molecule is being broken down into smaller molecules. But enzymes catalyse oxidation reactions, condensation reactions in which big molecules are built up from smaller ones, phosphorylation reactions (sticking phosphate groups onto molecules) and so on and so on. So don’t describe enzymes as being involved in breaking things down: some do of course but the vast majority work inside cells to catalyse a whole variety of reactions in metabolism.
Rates of enzyme-catalysed reactions can be affected by Temperature
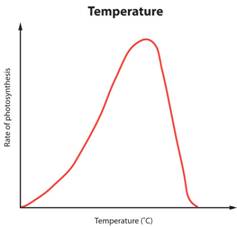
The temperature of the reaction has a significant effect on the rate of reaction. Look at the following graphs:
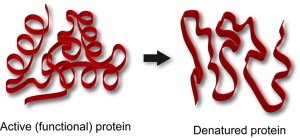
The pattern of this graph is characteristic of an enzyme catalysed reaction. At low temperatures the rate of reaction is low. This is because few enzyme-substrate complexes are formed per second as the enzyme and substrate molecules are moving around so slowly that they rarely collide. At temperatures above the optimum, the enzymes and substrate molecules will be moving very fast and so will be colliding all the time. So why is the rate so low? Well that is because high temperatures cause enzymes to denature. Remember enzymes are made of protein and proteins can have their 3D shape changed by high temperatures. If an enzymes’ 3D shape changes, the active site will change shape and if this happens the substrate cannot bind.
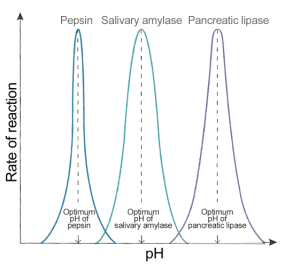
Rates of enzyme-catalysed reaction are also affected by pH
Enzymes tend to work in a very narrow band of pH values. pH is a measure of the acidity/alkalinity of a solution and most enzymes require an optimum pH to function well. The rate of reaction drops very rapidly on either side of the optimum pH simply because extremes of pH will denature enzymes. The acid or alkaline environment can break the bonds that hold the enzyme in its specific 3D shape. An enzyme with a changed shape cannot function as a catalyst if the substrate cannot bind to the active site and so the rate falls away rapidly either side of the optimum.



I am so thankful and grateful to you. This is the best site ever !!!
I’m pleased you like it. Keep working hard!
This is quite hard to answer. The how it increases the rate is actually quite complex…. When the substrate is bound to the active site (forming an enzyme-substrate complex) a strain is put onto the bonds in the substrate making it easier for the reaction to happen. This means there will be more reactions per second and so the rate of the reaction goes up. If you want to do some more research into how an enzyme molecule can speed up a reaction, try to find out about the induced fit model for how enzymes work. But you certainly don’t need this for IGCSE!!
Here is another student question from 3H.. “Can you state an example of how an enzyme increases the rate of a reaction?”
And the response is as follows…. Substrates moving too fast is never a problem. The problem is that at high temperatures above the optimum, the enzyme changes shape (denatures). If the active site has changed shape, it doesn’t matter how many times a substrate collides with it, an enzyme-substrate complex will never form. Hope this helps!
I was asked this question by a 3H student. “When the temperature is lower than the optimum, enzymes move around too slowly and they rarely collide. But if the temperature is higher, the enzymes and substrate molecules move very fast and always collide – why is this a problem when you want them to collide and bind together? Is it something to do with the substrates not being able to bind because of them moving too fast? If so then why: shouldn’t they both be moving at the same speed so they can catch up with each other?”
How do the different enzymes know to bond to certain substrates that fit their active site?
The bonding between S and E is quite complex. Substrates are held in the active site by weak inter-molecular forces called hydrogen bonds. So it is not enough for a substrate to be the correct shape to fit into the active site, it also has to have the correct properties to form these hydrogen bonds to hold it in place. Hope this helps……
So are there lots of different types of enzymes and if so what sort of sizes can they be?
There are as many different types of enzyme as there are reactions to catalyse. Each enzyme is specific to a particular reaction. Can you use Lock and Key theory to explain why this is?
How do enzymes get used in a reaction and not be used up?
Good question – I hope the description of Lock and Key theory that we covered in class answers this for you…. Once the products of the reaction have fallen out of the active site at the end of a reaction, the enzyme is exactly the same as it was at the start. So a new substrate can collide with the empty active site and it all starts again……
Can you use the same enzymes again for the same reaction because they don’t get used up and why don’t they get used up in the reaction, what happens?
Great question. Short answer is yes you can use enzymes over and over again as they don’t get used up in the reaction. Once the active site of the enzyme is empty when the products of one reaction fall out, a new substrate can then bind. Some enzymes can catalyse thousands of reactions per second so are very effective catalysts!
So does the active site shape or the enzyme change or is it both when they are denatured?
When an enzyme denatures, the whole enzyme molecule (which is made of protein) changes shape. But the part of the enzyme molecule that is important is the active site, because if its shape changes, the substrate molecule cannot fit into it and so the enzyme cannot function as a catalyst. I hope this helps……